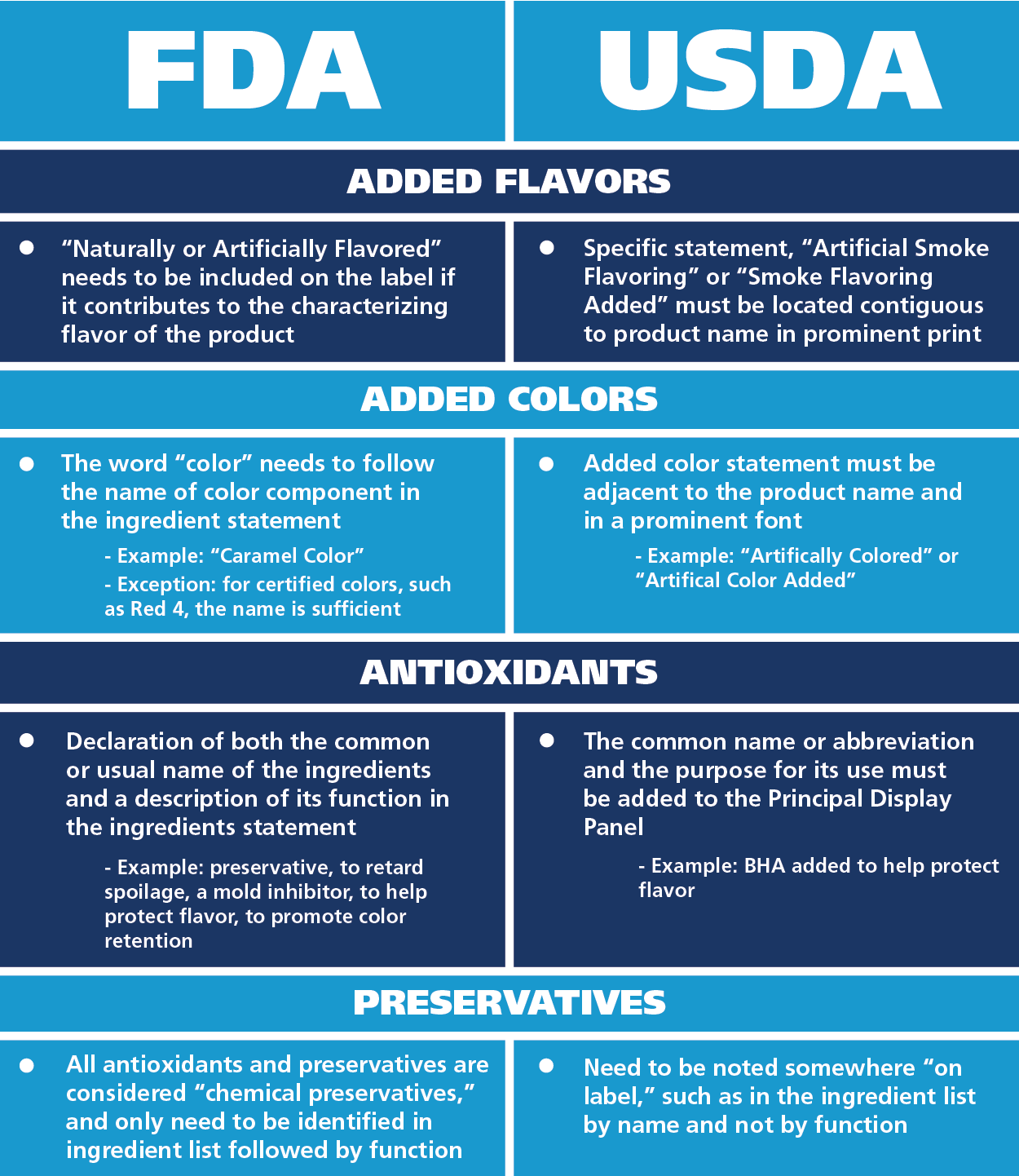
Fda Manufacturer Label Requirements . Section 21 cfr 820.80 (b) requires the inspection and. Web the fda labeling requirements cover a wide range of information, including: Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web fda drug labeling requirements & regulations: Web various sections of the qs regulation have an impact on labeling: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Packaging and labeling requirements for specific products.
from regulationlatest.blogspot.com
Web the fda labeling requirements cover a wide range of information, including: Packaging and labeling requirements for specific products. Web fda drug labeling requirements & regulations: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web various sections of the qs regulation have an impact on labeling: Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Section 21 cfr 820.80 (b) requires the inspection and.
Fda Regulations For Food Manufacturing
Fda Manufacturer Label Requirements Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Packaging and labeling requirements for specific products. Web fda drug labeling requirements & regulations: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Section 21 cfr 820.80 (b) requires the inspection and. Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web various sections of the qs regulation have an impact on labeling: Web the fda labeling requirements cover a wide range of information, including:
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Fda Manufacturer Label Requirements Packaging and labeling requirements for specific products. Web the fda labeling requirements cover a wide range of information, including: Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web (a) the label of a device in package form shall specify conspicuously the name and place of business. Fda Manufacturer Label Requirements.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Manufacturer Label Requirements Web the fda labeling requirements cover a wide range of information, including: Web fda drug labeling requirements & regulations: Web various sections of the qs regulation have an impact on labeling: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web labeling regulations pertaining to medical devices. Fda Manufacturer Label Requirements.
From abcnews.go.com
FDA Announces First Nutrition Label Change in 20 Years ABC News Fda Manufacturer Label Requirements Packaging and labeling requirements for specific products. Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web the fda labeling requirements cover a wide range of information, including: Section 21 cfr 820.80 (b) requires the inspection and. Web labeling regulations pertaining to medical devices are found in. Fda Manufacturer Label Requirements.
From www.nfpt.com
Understanding the Changes to the 2018 FDA Nutrition Label Fda Manufacturer Label Requirements Web the fda labeling requirements cover a wide range of information, including: Web fda drug labeling requirements & regulations: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Section 21 cfr 820.80 (b) requires the inspection and. Packaging and labeling requirements for specific products. Web various sections. Fda Manufacturer Label Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Manufacturer Label Requirements Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web fda drug labeling requirements & regulations: Packaging and labeling requirements for specific products. Web the fda labeling requirements cover a wide range of information, including: Web various sections of the qs regulation have an impact on labeling:. Fda Manufacturer Label Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Manufacturer Label Requirements Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Section 21 cfr 820.80 (b) requires the inspection and. Packaging and labeling requirements for specific products. Web the fda labeling requirements cover a wide range of information, including: Web various sections of the qs regulation have an impact. Fda Manufacturer Label Requirements.
From regulationlatest.blogspot.com
Fda Regulations For Food Manufacturing Fda Manufacturer Label Requirements Web the fda labeling requirements cover a wide range of information, including: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Section 21 cfr 820.80 (b) requires the inspection and. Web fda drug labeling requirements & regulations: Packaging and labeling requirements for specific products. Web labeling regulations. Fda Manufacturer Label Requirements.
From www.onlinelabels.com
What are the FDA Labeling Requirements for Cosmetic Products? Fda Manufacturer Label Requirements Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Section 21 cfr 820.80 (b) requires the inspection and. Web various sections of the qs regulation have an impact on labeling: Packaging and labeling requirements for specific products. Web fda drug labeling requirements & regulations: Web labeling regulations. Fda Manufacturer Label Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Manufacturer Label Requirements Section 21 cfr 820.80 (b) requires the inspection and. Packaging and labeling requirements for specific products. Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web the fda labeling requirements cover a wide range of information, including: Web various sections of the qs regulation have an impact. Fda Manufacturer Label Requirements.
From greatlakeslabel.com
New 2020 (NLEA) Food Label Requirements Great Lakes Label Fda Manufacturer Label Requirements Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web various sections of the qs regulation have an impact on labeling: Packaging and labeling requirements for specific products. Web fda drug labeling requirements & regulations: Web the fda labeling requirements cover a wide range of information, including:. Fda Manufacturer Label Requirements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Manufacturer Label Requirements Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web various sections of the qs regulation have an impact on labeling: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web fda drug labeling. Fda Manufacturer Label Requirements.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Fda Manufacturer Label Requirements Web the fda labeling requirements cover a wide range of information, including: Section 21 cfr 820.80 (b) requires the inspection and. Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web fda drug labeling requirements & regulations: Web (a) the label of a device in package form. Fda Manufacturer Label Requirements.
From www.slideshare.net
Fda requirements for device labeling Fda Manufacturer Label Requirements Section 21 cfr 820.80 (b) requires the inspection and. Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Web various sections of the qs regulation have an impact on labeling: Packaging and labeling requirements for specific products. Web the fda labeling requirements cover a wide range of. Fda Manufacturer Label Requirements.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Fda Manufacturer Label Requirements Section 21 cfr 820.80 (b) requires the inspection and. Packaging and labeling requirements for specific products. Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web various sections of the qs regulation have an impact on labeling: Web labeling regulations pertaining to medical devices are found in. Fda Manufacturer Label Requirements.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Fda Manufacturer Label Requirements Packaging and labeling requirements for specific products. Web the fda labeling requirements cover a wide range of information, including: Web fda drug labeling requirements & regulations: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web labeling regulations pertaining to medical devices are found in the following. Fda Manufacturer Label Requirements.
From fra.animalia-life.club
Iso 15223 1 Symboles Fda Manufacturer Label Requirements Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Packaging and labeling requirements for specific products. Section 21 cfr 820.80 (b) requires the inspection and. Web various sections of the qs regulation have an impact on labeling: Web the fda labeling requirements cover a wide range of. Fda Manufacturer Label Requirements.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Manufacturer Label Requirements Web the fda labeling requirements cover a wide range of information, including: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Packaging and labeling requirements. Fda Manufacturer Label Requirements.
From www.usimports.us
Food Labels Must Comply with FDA Ingredient Statement Requirements Fda Manufacturer Label Requirements Section 21 cfr 820.80 (b) requires the inspection and. Packaging and labeling requirements for specific products. Web fda drug labeling requirements & regulations: Web various sections of the qs regulation have an impact on labeling: Web (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. Web labeling regulations. Fda Manufacturer Label Requirements.